-
Protocols
Experimental protocols are defined within the platform and encompass study conduct, subjects, and instrument settings. All of this information follows a data set from raw form through to all downstream analyses.
-
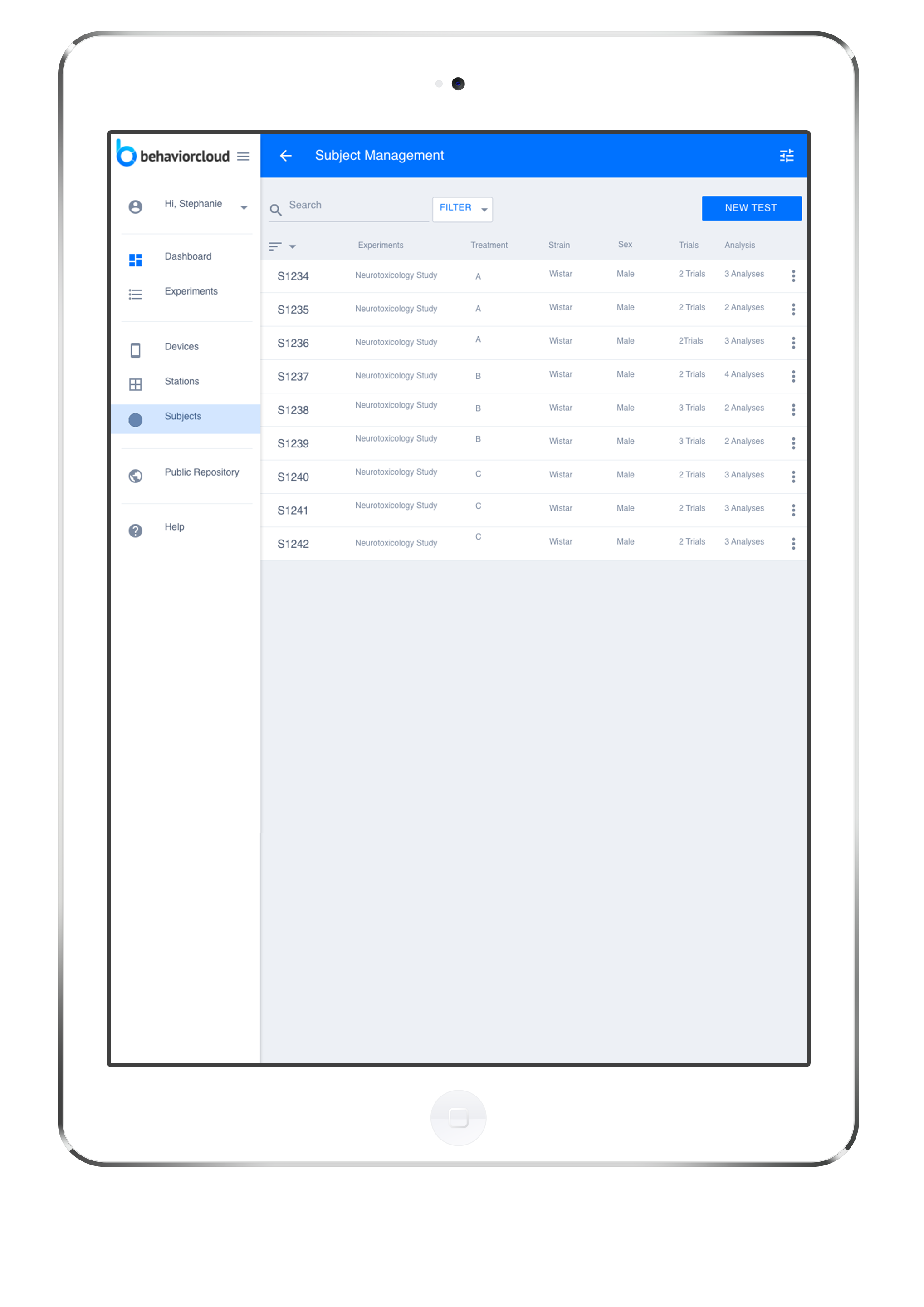
Subject Management
Our subject management database lets you keep track of experimental subjects and their corresponding meta-data, while maintaining a separation from any treatment identifying information during the data collection phase.
-
Study Reports
The built-in lab notebook feature makes it easy to keep all raw data and study-related information in one searchable, traceable repository.
-
Audit Logs and Versioning
Changes to datasets and documents are audit logged and all versions are maintained in archival so every study is traceable and reproduceable.